It seems like a basic enough question, but when you realize that energy can’t be created or destroyed, things get less obvious fast. The knee-jerk reaction is to say that the light gets absorbed into the walls, but how? What does it even mean to say that light gets “absorbed?”
It seems like a basic enough question, but when you realize that energy can’t be created or destroyed, things get less obvious fast. The knee-jerk reaction is to say that the light gets absorbed into the walls, but how? What does it even mean to say that light gets “absorbed?”
Photon: The Particle That Didn’t Exist
If you’ve read a pop-sci book here or there, you’ve probably heard of the photon: the little bundle of light. It carries light from here to there. It bounces back and forth between electrons and protons, carrying the electromagnetic force and holding the atom together.
The particle you’re imagining is a lie.
Don’t get me wrong, the photon is a real thing, but you’re not going to get any genuine understanding of what’s going on here unless you banish that word “particle” from your mind.
Instead, we’re going to take a journey back in time, and talk about the centuries old concept of the electromagnetic field. Don’t worry, we’ll get back to the bizarre world of quantum mechanics soon enough.
Just What is this “Field?”
Put simply, in physics, a field is another way of saying that we can assign every point in space some numerical value: a potential. For example, the electric field assigns every point in space some quantity that we would measure as “energy per unit charge.”
Fields tell things where to go. An object will naturally slide from high energy to low energy, like a rock down a hill. The “energy per unit charge” tells a charged object how much energy is at each point in space for a particle with that amount of charge.
If a particle has the opposite charge, everything gets flipped upside down. The hill becomes a valley.
We call it the electromagnetic field, instead of the electric field, because the two are related. A magnetic compass heading north will end up running in circles around an electric wire.
But we also have to remember that charged objects change the field too.
A positive charge becomes the peak of a hill to other positive charges, and they slide away from it. But it becomes the center of a valley to the negative charges, which end up sliding toward it.
Charges don’t change the landscape of this field immediately. If a positive charge suddenly shifts it’s position, the field around it will ripple outward and reorganize itself.
And so, if a charged particle starts vibrating, it will send out waves through this field. And if a charged particle gets hit by this wave, it will start vibrating as well, since the hills and valleys are constantly shifting position.
That is what light is, a wave in the electromagnetic field. The only problem is, real particles don’t quite look like the objects we imagined here. In fact, it turns out, they’re also waves.
Heisenberg’s Omnipresent Electron
Enter the quantum: the elusive sort-of wave, sort-of particle, that defies all common sense.
Here’s where I’m going to give you a tool that should help you think about quanta without having an aneurysm. Once again, I’m going to ask you to throw out that word “particle.” Just forget you ever even heard it. Take that word, and mentally replace it with “unit” every time you hear it.
Now picture a world with no electrons. Instead, the world is filled with an omnipresent electron field. This field is everywhere. It has peaks and valleys, just like the electromagnetic field.
This field is measured in units of electron mass, electron charge, and electron spin, and it carries additional energy in the form of electron momentum.
The momentum of this field is measured as frequency, which is how fast waves are vibrating in the field. If it’s vibrating fast, the momentum’s high. If it’s vibrating slowly, the momentum’s low.
To measure this momentum perfectly, we’d need the waves to be perfectly evenly spaced throughout the entire universe. The electron would be everywhere and nowhere.
Alternatively, you can smoosh the waves together into the form of a hill with a few ripples around it. Now you have a better idea of where the “electron” is, but the waves aren’t evenly spaced, so we know less about it’s momentum.
Here’s a rough idea of what this looks like, thanks to Wikipedia: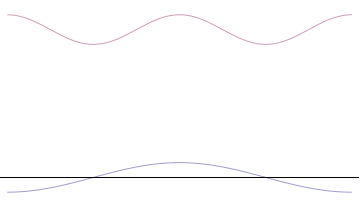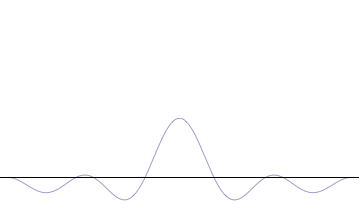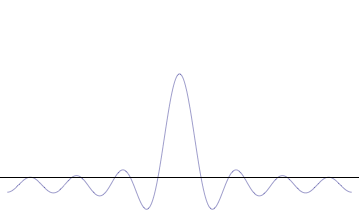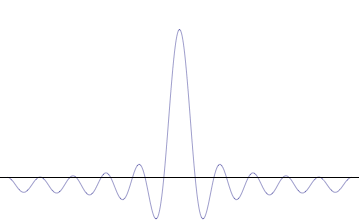
This is how we can gather together some energy from the electron field and put it mostly in one place, then call it an electron for our own convenience.
Energy gets transferred to and from this electron field in discrete units that we call electrons. That’s the only thing about the electron field that really brings particles to mind, but it’s a loose analogy and, in my opinion, it confuses things more than it helps.
And this is, more or less, how every “particle” in the universe works. It’s actually just energy carried as vibrations in a quantum field.
Rethinking the Photon
And so it goes for the photon. A photon is a ripple in the electromagnetic field, not much different from the concept of the electromagnetic wave we already talked about.
The only significant difference quantum mechanics introduces, here, is the idea that energy gets extracted from and introduced to the electromagnetic field in discrete units. We can’t add half a photon unit to the electromagnetic field, or pull half a photon unit out of it.
But these ripples still travel through the field like waves. They spread out, interfere with each other, and mix together.
So, now that we understand electrons and photons aren’t much like particles at all, we can finally talk about how a photon gets absorbed into the wall.
When a Photon Hits Your Wall
Lets start with the atoms in your wall.
As you already know, an atom is made up of negatively charged electrons surrounding a positively charged nucleus, but if you’re picturing electrons “orbiting” or “buzzing” around the nucleus, that’s not how it works.
Think of the nucleus as a valley in the electromagnetic field that the electron wants to slide into. But remember, the electron isn’t a tiny charged ball bearing, it’s a ripple in the electron field. Since it’s negatively charged, it also creates hills in the electromagnetic field wherever it goes.
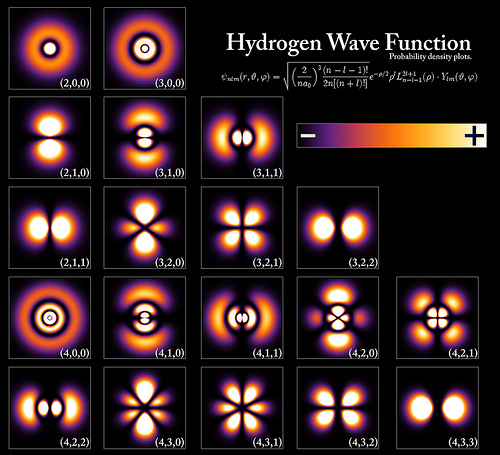
Remember how the momentum of the electron field is measured by how fast the ripple is vibrating? These ripples can interfere with each other, cancelling each other out in some places, and reinforcing each other in others. This causes electrons to coalesce around nuclei in interesting shapes, like this:
And so, the shape of the electron ripple will change if it’s momentum changes. But only certain levels of momentum are possible. The others would simply cause the electron to interfere with itself and vanish out of existence, an impossibility. And so, we say, there are only certain “energy levels” that the electron is capable of having.
When the photon ripples up against one of these atoms, there are a few things that can happen. It can reflect off of the atom, it can ripple right through it, or it can be absorbed.
We’ll focus on absorption.
The electron can only absorb the photon if it extracts enough energy from the electromagnetic field to jump up to a higher energy level. In other words, a ripple in the electromagnetic field (photon) becomes an increase in the vibration of the electron field (momentum).
When the electron falls back down to a lower energy level, this energy can get transferred back to the electromagnetic field as another photon, but this isn’t always what happens.
Instead, the momentum of the electron can be transferred to the momentum of the atom as a whole. This causes the atom itself to vibrate, which we call heat.
So, where does the light go when we shut it off? It changes from vibrations in the electromagnetic field, to vibrations in the electron field, and then to vibrations in the atomic or molecular field, which we call heat.
The Scientific Consensus On Climate Change